Naturally occuring white calcium sulphate dihydrate
(CaSO4·2H2O)
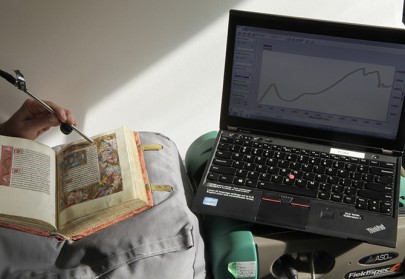
Abundant in nature, gypsum was not commonly used as a white pigment, but it was often the main component of the preparatory layer for gold leaf. It could also be used as part of the manufacturing process of lake pigments and it is often detected in manuscripts where pink hues were obtained with organic colourants.
Gypsum can easily be identified by the characteristic absorbance bands in the near-infrared portion of its reflectance spectrum.